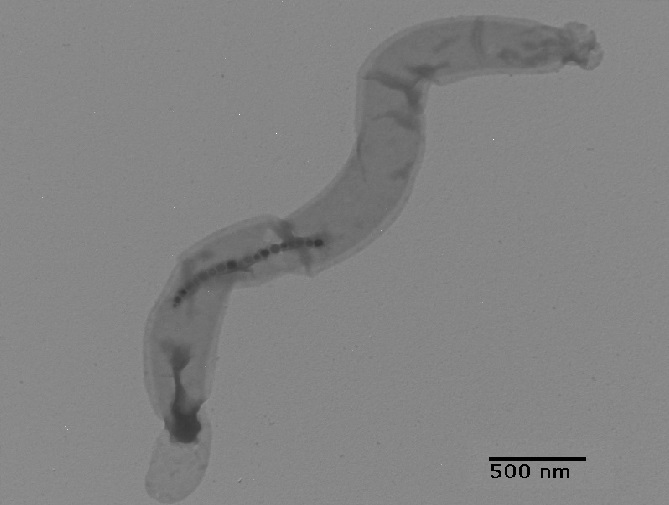
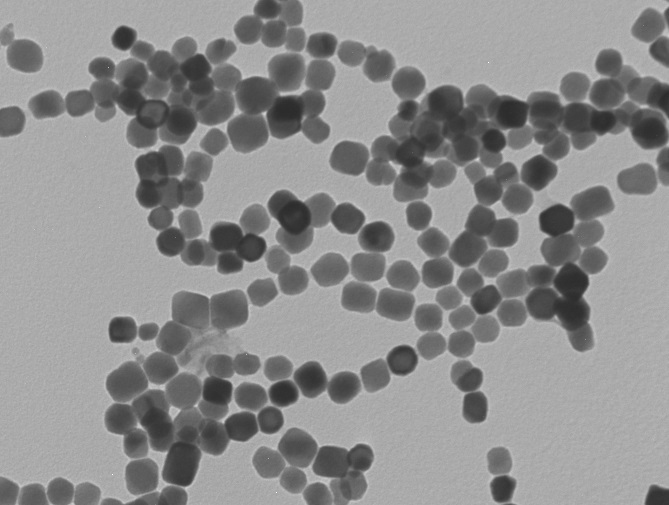
Biofilm
Microbes have long existed for billions of years and they form approximately 60% of the biological mass on earth. Microbial biofilms play important roles in the environment and they also have great impacts on human health. During biofilm formation, microbes exhibit biochemical pathways that may cause product contamination, energy losses, medical infection, and material corrosion. Most mineral depositions, such as calcium, are found on surfaces of bacterial colonies in forms of crystals. It is important to understand the formation process of bacterially precipitated minerals, and ultimately control microbial growth and biofilm formation under an ideal circumstance.
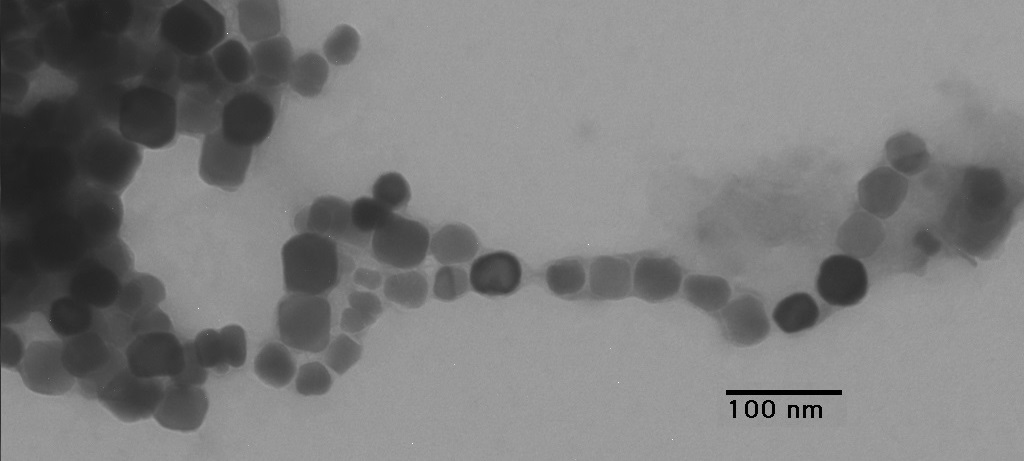
During early biofilm formation, individual bacterium spreads out chemotactically and communicates with other surrounding bacteria through the process of quorum sensing. This method of communication is dependent on certain aspects of physiology of the bacteria as a function of population and density of its own species. It is discovered that the role of quorum sensing is dependent on the amount of nutrients present. Thus biofilm architecture can be controlled by different combination of nutrient components.
Formation of biofilm involves several stages, beginning with formation of a conditioning layer that induces initial absorption of bacterial to the surface. A suitable environment for bacterial growth involving proteins or carbohydrates is created during this stage. The next stage involves in bacterial growth and reproduction, followed by formation of an extracellular matrix which allows bacterial attachment. The last stage consists of a complex structure that continuously supplies nutrients for bacterial metabolism and waste product removal. The growth rate of the four stages is dependent on amount of nutrient supply, structure of matrix, medium composition, as well as bacterial population.