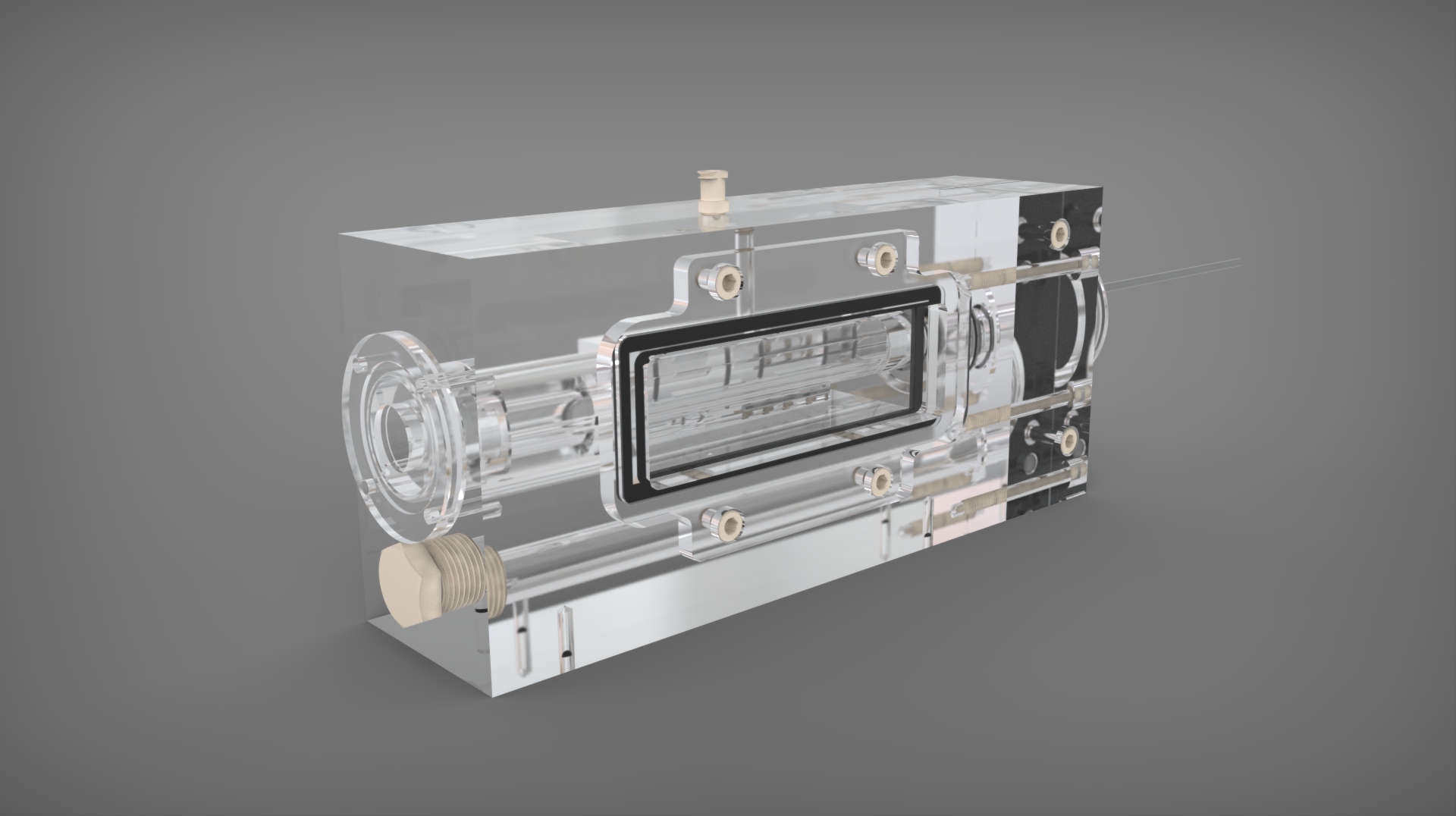
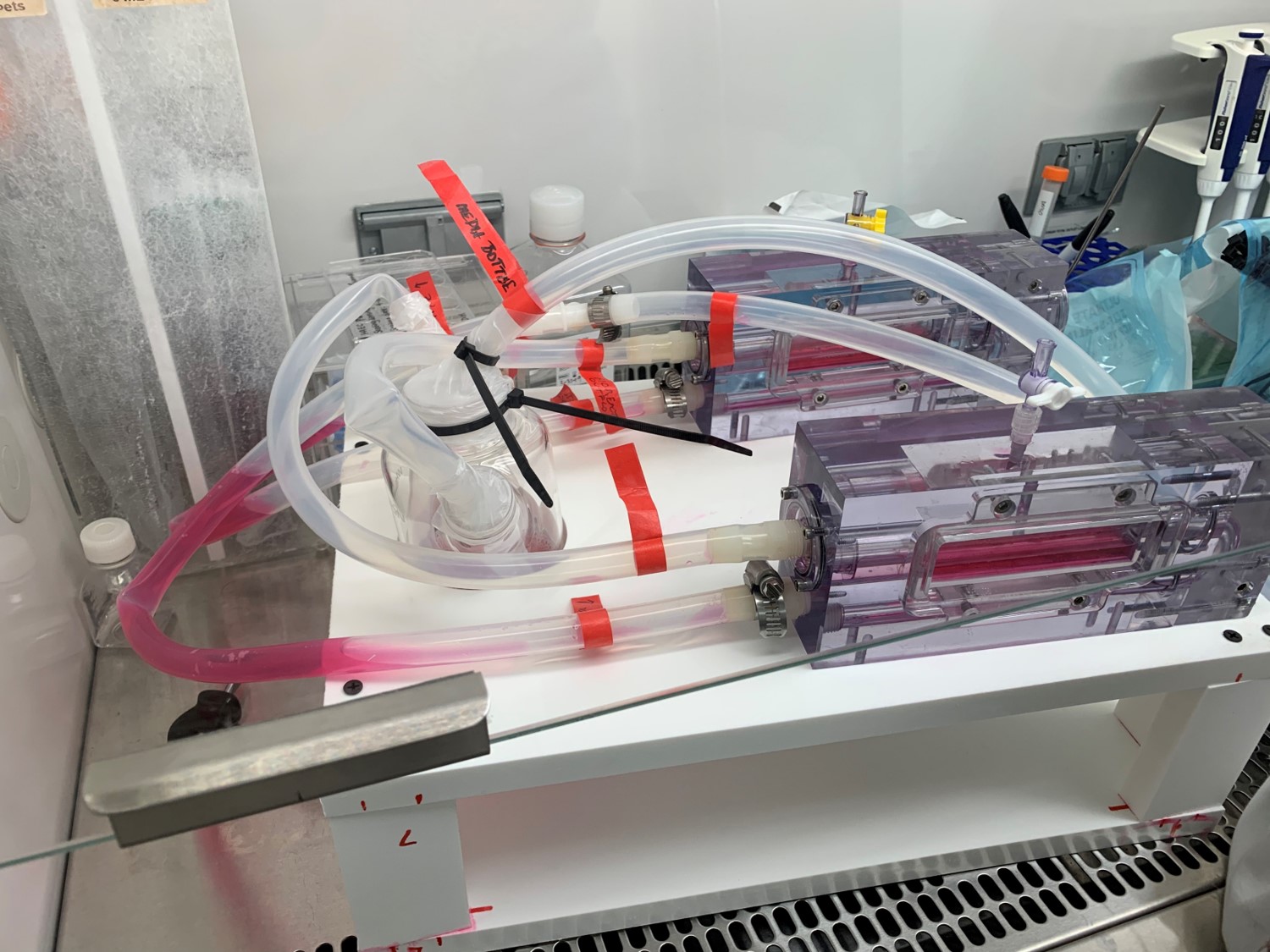
Pulsatile Flow in Bioreactor
Congenital heart disease and calcific aortic valve disease are the most prevalent heart valve
anomalies. Each year, approximately 300,000 patients undergo valve replacement surgery worldwide
and, as the average age of the population increases, it is projected to reach 850,000 by the year
2050. The preferred method of treating valve disease is to repair the existing diseased valves
wherever possible; however, in advanced stages of disease or anomalies, the only solution is a
complete replacement. Currently two types of artificial valves exist for replacement: mechanical
and bioprosthetic. Mechanical valves have a longer lifetime than bioprosthetic valves, but have
a higher long-term risk for blood clots leading to stroke or arterial thrombosis. Thus, patients
require long term anticoagulation therapy to prevent thrombosis, which may lead to serious bleeding
complications if not well controlled. Bioprosthetic valves, on the other hand, exhibit limited
durability, in which patients usually require a reoperation 10-15 years post implantation to replace
the valve. Furthermore, bioprosthetic valves are prone to accelerated calcification, especially in
children. For pediatric patients with CHD, existing replacement devices are not a viable option due
to their inability to accommodate somatic growth. As the child grows, the implanted valve will be
outgrown, requiring surgical replacement with a larger valve. As a result, pediatric patient mortality
and morbidity are substantially augmented relative to adults due to multiple major surgeries in the
patients who already have severe heart problems. With the current limitations of treatment options, the
probability of success for valve diseases leveraging a tissue engineering approach is high. Researchers
have determined that flow, stretch, and flex are important mechanical modes of stimuli for tissue
construct. To achieve a suitable physiologically relevant environmental condition for tissue growth, a
flow mediated bioreactor has been designed to aid researchers in developing engineered valve tissue
constructs for the heart.
Project Aims
Our specific aims of this project include: To verify or establish a similar flow profile with an oscillatory shear index (OSI) range for 3D tissue engineering that promotes valve tissue growth on a suitable scaffold, in addition to successful valve tissue production that can eventually be incorporated as treatment for valve replacement or repair. Successful completion of this work entails two important milestones: (1) Verification of tissue phenotype derived from bioreactor experiments after conditioned flow and scaffold seeding, and (2) Results of assessments (histology and mechanical testing) in the grown tissue exhibit similar properties to a native valve tissue.